Abstract
Heme is a byproduct of hemolysis released into the circulation. Heme oxygenase-1 (HO-1), the rate limiting enzyme breaks down heme from damaged or senescent red blood cells, exerting antioxidant and anti-inflammatory cytoprotective effect, especially in sickle cell disease (SCD). HO-1 is regulated by heme through the NRF2 transcription factor. Placental Growth Factor (PlGF) is an angiogenic growth factor secreted by proliferating erythroblasts during normal development, which we previously have found is also activated by heme, secondarily regulating expression of the vasoconstrictor endothelin-1 that promotes pulmonary hypertension. Because hemolysis contributes to organ damage in SCD, our objective was to assess the multi-organ expression of HO-1 and PlGF in adult sickle (SS) mice as a marker of heme uptake and vascular injury respectively, and to identify most at risk organs during acute elevation of circulating extracellular heme. Furthermore, we investigated the transcriptional response of HO-1 and PlGF in different organs in C57BL/6J wild type (WT) and Nrf2 null mice following acute exposure to heme to understand the molecular pathways involved.
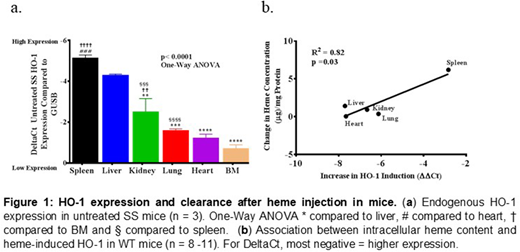
HO-1 expression varied dramatically by organ in untreated SS mice, by real time PCR. Using Delta Ct (most negative=higher expression), there was heterogeneity in expression of HO-1 among different organs (p<0.0001, One-way ANOVA). Spleen (-5.1±0.1), liver (-4.3±0.0) and kidney (-2.5±0.6) showed higher expression compared to other organs (Fig. 1a). PlGF expression on the other hand, was higher in the heart (2.9±0.3), lung (3.1±0.2) and kidney (5.0±0.6) (p<0.0001, One-way ANOVA). To mimic acute hemolysis, 50µmoles/kg bodyweight of heme was infused into SS mice, and organs were harvested 3 hours later. The expression of HO-1 and PlGF was strongly induced (p<0.0001, One-way ANOVA), augmenting the previous pattern observed in untreated SS mice. Surprisingly, we observed a submaximal response to HO-1 induction in the liver of SS mice injected with hemin compared to control. We confirmed the variability in organ response to heme in WT mice injected intravenously with saline or hemin (120µmoles/kg body weight), followed by harvest of the spleen, liver, kidney, lung and heart 3 hours after injection. Heme oxygenase-1 mRNA levels increased sharply in organs from WT mice that received hemin compared with saline control: liver (207-fold), heart (201-fold), kidney (101-fold), lung (69-fold) and spleen (7-fold). This induction was largely Nrf2-dependent, with 59-78% lower response in Nrf2 null mice in every organ except kidney. The 101-fold induction of HO-1 in the kidney was essentially Nrf2 independent. Next, we measured heme content of each organ 3 hours after injection of heme. The organs with highest heme induction of HO-1 appeared to clear heme more rapidly (R2=0.82, p=0.03) (Fig. 1b) and interestingly, the expression of the heme importer Hrg1 was also induced significantly in these organs (R2=0.82, p=0.004). PlGF expression in WT mice was induced by heme more strongly in the heart, lung and kidney (parallel to SS mice) and was approximately 50% Nrf2-dependent in all organs sampled.
The reticulo-endothelial organs have been shown by others to take up free heme avidly, but these are the first data to demonstrate widely ranging differences in heme response among other organs, and the degree of HO-1 induction in the heart is surprisingly comparable to liver and kidney. Also, pharmacological induction of HO-I has been proposed as a therapy in SCD. Our result showing submaximal induction of HO-1 in SS mice suggests suboptimal expression of HO-1 in SCD. This is consistent with published benefit in sickle cell mice of HO-1 augmentation by gene transfer or Nrf2-inducing agents. Understanding the mechanism of organ-specific response to heme and HO-1/PGF induction could justify the introduction of early therapy to mitigate heme-mediated injury to organs and tissues in SCD and slow progression to organ failure, especially in the aging population of adults with SCD.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal